The Endocrine Society is dedicated to providing the field of endocrinology with timely, evidence-based recommendations for clinical care and practice. We continually develop new guidelines and update existing guidelines to reflect evolving clinical science and meet the needs of practicing physicians. We strive to adhere to national and international quality standards and employ rigorous methodology. We continually make improvements on our protocols to ensure best practices. The clinical practice guidelines development program is overseen by the clinical guidelines committee (CGC).
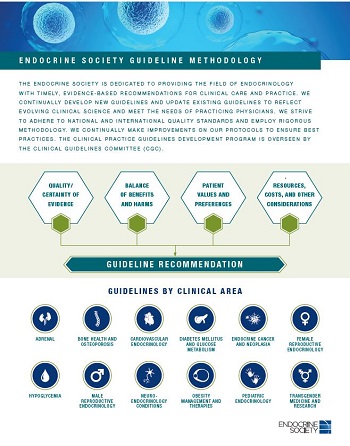 The Society’s guideline development process follows the GRADE methodology (Grading of Recommendations Assessment, Development and Evaluation). GRADE encompasses a set of standardized and transparent procedures for assessing the quality of available evidence, the relative importance of outcomes (expected benefits and potential harms), the relative utility of alternative management strategies, and patient and public preferences/values. GRADE also provides a structure for translating said evidence and values into user-friendly recommendations. Gain an overview our methodology steps here or in the adjacent graphic.
The Society’s guideline development process follows the GRADE methodology (Grading of Recommendations Assessment, Development and Evaluation). GRADE encompasses a set of standardized and transparent procedures for assessing the quality of available evidence, the relative importance of outcomes (expected benefits and potential harms), the relative utility of alternative management strategies, and patient and public preferences/values. GRADE also provides a structure for translating said evidence and values into user-friendly recommendations. Gain an overview our methodology steps here or in the adjacent graphic.
Some of the Society’s clinical practice guidelines also include Ungraded Good Practice Statements (3). This unclassified clinical guidance can include expert opinion statements on good practice, references to recommendations made in other guidelines, and observations on preventive care and shared-decision-making.
 Guideline recommendations include the relevant population, intervention, comparator and outcome. When further clarification on implementation is needed, we include Technical Remarks. These provide supplementary information such as timing, setting, dosing regimens, and necessary expertise.
Guideline recommendations include the relevant population, intervention, comparator and outcome. When further clarification on implementation is needed, we include Technical Remarks. These provide supplementary information such as timing, setting, dosing regimens, and necessary expertise.
All recommendations are followed by a synopsis of the evidence which underpins it. This includes statements on patients’ values and preferences, the balance of benefits and harms, and minority opinions, where relevant. Read our full Guideline Methodology here or by visiting the adjacent document.
 There are many sources of guidance on conflict of interest (COI) as it relates to establishing guideline writing groups and the production of guidelines themselves. The Endocrine Society’s Clinical Guidelines Committee (CGC) [formerly Clinical Guidelines Subcommittee] finds guidance provided by the Guidelines International Network (G-I-N) to be clear and concise, and a document published by G-I-N in 20151 forms the primary basis of this COI policy. However, standards from the Institute of Medicine’s (now the National Academy of Medicine’s) Clinical Practice Guidelines We Can Trust2, other similar documents, and the COI policies of other medical societies have also informed the content of this policy. The Society’s COI rules for the development of clinical practice guidelines are spelled out here and in the adjacent document.
There are many sources of guidance on conflict of interest (COI) as it relates to establishing guideline writing groups and the production of guidelines themselves. The Endocrine Society’s Clinical Guidelines Committee (CGC) [formerly Clinical Guidelines Subcommittee] finds guidance provided by the Guidelines International Network (G-I-N) to be clear and concise, and a document published by G-I-N in 20151 forms the primary basis of this COI policy. However, standards from the Institute of Medicine’s (now the National Academy of Medicine’s) Clinical Practice Guidelines We Can Trust2, other similar documents, and the COI policies of other medical societies have also informed the content of this policy. The Society’s COI rules for the development of clinical practice guidelines are spelled out here and in the adjacent document.
Funding for the development of guidelines is provided by the Endocrine Society. No other entity provides financial or other support.